
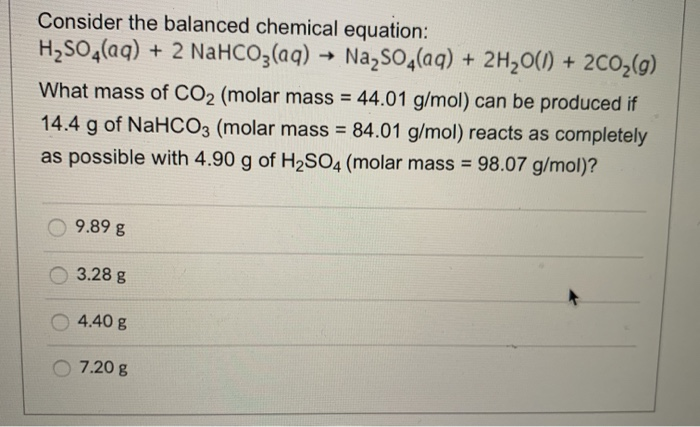
Because baking soda will absorb musty smells, it has become a reliable method for used book sellers when making books less malodorous. It has weak disinfectant properties, and it may be an effective fungicide against some organisms. Sodium bicarbonate is also used to delay combustion reactions by releasing CO 2 and H 2O when heated, both of which are flame retardants. The effect is caused by the thermal decomposition, which produces carbon dioxide gas to produce a long snake-like ash as a combustion product of the other main component, sucrose. Sodium bicarbonate is one of the main components of the common "black snake" firework.
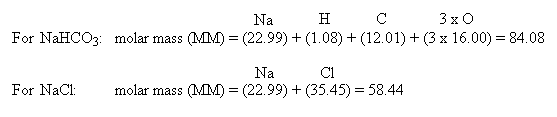
Baking soda is alkaline the acid used in baking powder avoids a metallic taste when the chemical change during baking creates sodium carbonate. Many forms of baking powder contain sodium bicarbonate combined with calcium acid phosphate, sodium aluminium phosphate, or cream of tartar. Baking powder īaking powder, also sold for cooking, contains around 30% of bicarbonate, and various acidic ingredients which are activated by the addition of water, without the need for additional acids in the cooking medium. Since the reaction occurs slowly at room temperature, mixtures (cake batter, etc.) can be allowed to stand without rising until they are heated in the oven. Additionally, in the absence of acid, thermal decomposition of sodium bicarbonate also produces sodium carbonate, which is strongly alkaline and gives the baked product a bitter, "soapy" taste and a yellow color. When used this way on its own, without the presence of an acidic component (whether in the batter or by the use of a baking powder containing acid), only half the available CO 2 is released (one CO 2 molecule is formed for every two equivalents of NaHCO 3). Heat can also by itself cause sodium bicarbonate to act as a raising agent in baking because of thermal decomposition, releasing carbon dioxide at temperatures above 80 ☌ (180 ☏), as follows: 2 NaHCO 3 → Na 2CO 3 + H 2O + CO 2 Baking soda may be used together with sourdough, which is acidic, making a lighter product with a less acidic taste. The acid–base reaction can be generically represented as follows: NaHCO 3 + H + → Na + + CO 2 + H 2OĪcidic materials that induce this reaction include hydrogen phosphates, cream of tartar, lemon juice, yogurt, buttermilk, cocoa, and vinegar. When it reacts with acid, carbon dioxide is released, which causes expansion of the batter and forms the characteristic texture and grain in cakes, quick breads, soda bread, and other baked and fried foods. In cooking, baking soda is primarily used in baking as a leavening agent. The modern chemical formulas of these compounds now express their precise chemical compositions which were unknown when the name bi-carbonate of potash was coined (see also: bicarbonate).
The prefix bi in bicarbonate comes from an outdated naming system predating molecular knowledge in reference to the two molar equivalents of carbon dioxide (known as carbonic acid in the ancient chemistry language) that potassium hydrocarbonate/bicarbonate releases upon decomposition to (di)potassium carbonate and to potassium oxide (potash). The word saleratus, from Latin sal æratus (meaning "aerated salt"), was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate. Abbreviated colloquial forms such as sodium bicarb, bicarb soda, bicarbonate, and bicarb are common. and in many northern/central European countries it is called Natron. The term baking soda is more common in the United States, while bicarbonate of soda is more common in Australia, United Kingdom and Ireland. And don’t forget to put the unit g/mol to your final calculated molar mass.Because it has long been known and widely used, the salt has many different names such as baking soda, bread soda, cooking soda, and bicarbonate of soda and can often be found near baking powder in stores.First solve the brackets, then multiplications and at last do the final addition. Always follow the calculation order to avoid any mistakes in calculation.But all these units (i.e g/mol, grams/mole and g/mole) are the same. In some books, you may see the unit of molar mass as grams/mole or g/mole.I hope you have understood the short and simple calculation for finding the molar mass of Na2CO3.

Hence the Molar mass of Na2CO3 is 105.988 g/mol. So, Molar mass of Na2CO3 = Molar mass of 2 Sodium (Na) atoms + Molar mass of 1 Carbon (C) atom + Molar mass of 3 Oxygen (O) atoms.

You can see that in Na2CO3, there are 2 Sodium atoms, 1 Carbon atom and 3 Oxygen atoms. Now, to calculate the molar mass of Na2CO3, you just have to add the molar mass of all the individual atoms that are present in Na2CO3.


 0 kommentar(er)
0 kommentar(er)
